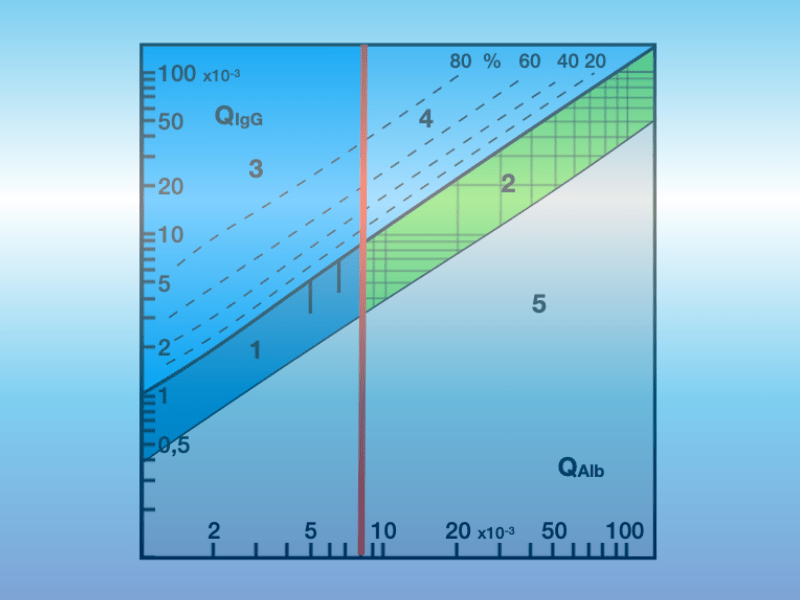
Kappa-free light chains in cerebrospinal fluid for the diagnosis of multiple sclerosis
A new protein known as κappa-free light chains (κ-FLC) in cerebrospinal fluid (CSF) has been identified as a biomarker for the early prognosis of individual disease progression in multiple sclerosis (MS). The kappa type of FLCs, which circulate predominantly as monomers (22 kDa) in the blood and cerebrospinal fluid, are formed in excess by [...]