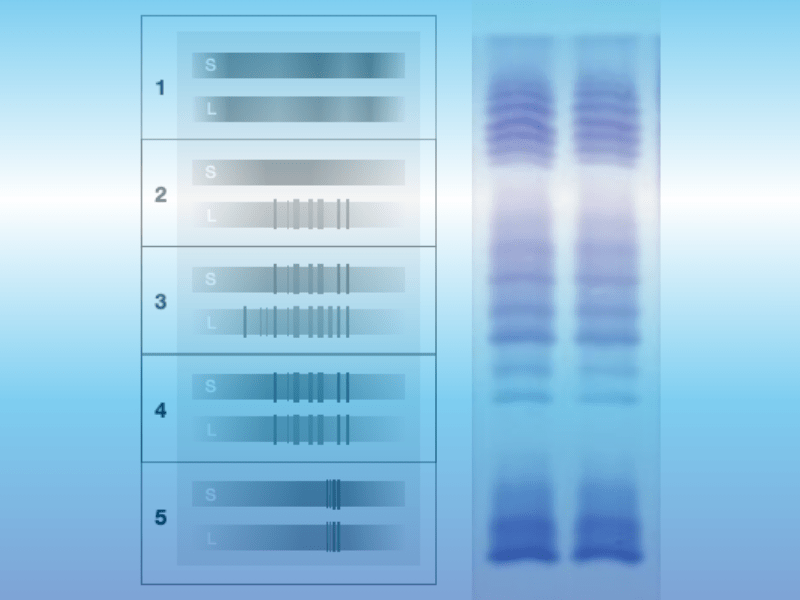
Oligoclonal bands: Quality control for IgG-specific oligoclonal bands
In addition to cell number, differential cell image, glucose-lactate total protein determination and the Reiber diagrams for albumin, IgG, IgA and IgM, the diagnosis of oligoclonal bands (OKB) is one of the central analytical methods in liquor diagnostics. The qualitatively reliable detection of oligoclonal IgG bands in CSF by means of isoelectric focusing (IEF) [...]