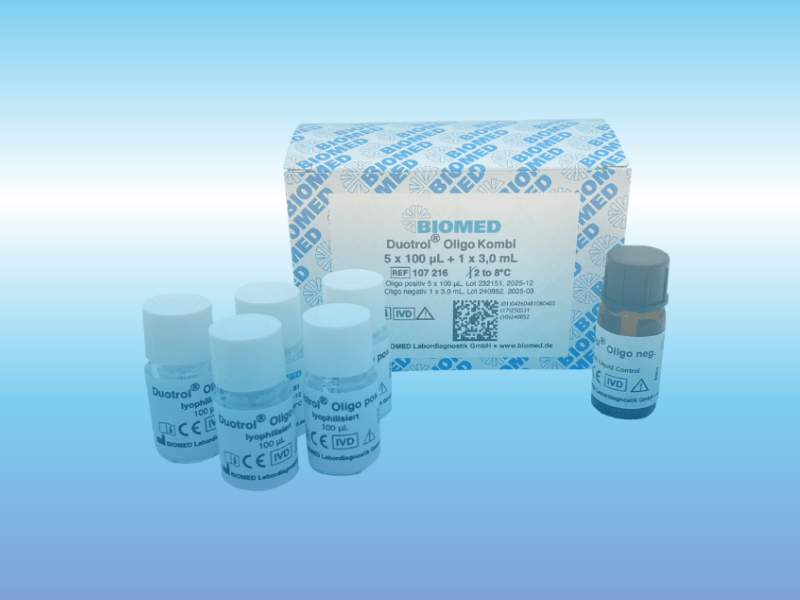
CSF quality control – our new Duotrol® controls for CSF diagnostics
Our well-established control for CSF diagnostics, Duotrol® CSF Liquor, is now reinforced by the new accuracy control Duotrol® CSQ Advanced, which replaces the previous Duotrol® CSQ Liquor. In addition to the standard parameters of CSF diagnostics, it offers values for ferritin in CSF for the detection of cerebral hemorrhages. Furthermore, by lowering the values [...]