Quality control in CSF diagnostics – Standard or economy-based? At the end of February 2024, our biologist and medical device consultant Dr. Dagmar Michel conducted an interview on this topic with Prof. Dr. Dr. Manfred Uhr, Head of the Clinical Laboratory at the Max Planck Institute of Psychiatry in Munich.
Prof. Manfred Uhr is a specialist in neurology, psychiatry and psychotherapy and head of the clinical laboratory at the Max Planck Institute of Psychiatry in Munich. As a member of the expert group for qualitative laboratory medical examinations of the German Medical Association and EQAS Expert at the EQA Organization Instand e.V., he gives an overview of the challenges in quality assurance of CSF and the quality of laboratory analyses.

Prof. Dr. Dr. Manfred Uhr, specialist in neurology, psychiatry and psychotherapy and Head of the Clinical Laboratory of the Max Planck Institute of Psychiatry in Munich
The field of in vitro diagnostics continuously strives to find innovative solutions for constantly changing requirements in the laboratory, and to implement suitable analytics as well as tests for reliable detection. Particular attention is given to the validity of the measurement results for the benefit of the patient. The update of RiliBÄK (Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations) in May 2023 is now also including the specifications for pre-analytics. In your opinion, what is currently changing regarding internal and external quality assurance and what do you think should change in the future?
Prof. Uhr:
Pre-analytics plays a very important role in CSF diagnostics. However, this relevance has no yet been adequately reflected in the RiliBÄK. Corresponding specifications are currently being prepared. Examples include the selection of tube material and the ratio of sample volume to tube volume. The time factor from sample collection to actual measurement also plays a not insignificant role for some CSF parameters. This factor should not be neglected when sending in samples.
What advantages do you see in the use of test-independent control materials, the application of which is recommended in DIN 15189:2023 for accredited medical laboratories?
Prof. Uhr:
The use of sample material as control material as well as EQA material should be carried out in accordance with the general selection of the RiliBÄK and should be as similar as possible to the material to be tested. Meaning, that the selected concentration range should be relevant for the clinical decision, and that the measurements should be taken in the correct matrix, e.g cerebrospinal fluid. This has not yet been sufficiently implemented for control material and, depending on the provider, for EQA material. Test-independent control material from third-party providers can offer advantages if it meets the above-mentioned requirements.
In your opinion, which new biomarkers are useful in CSF diagnostics for reliable results?
Prof. Uhr:
There are certainly several, and it is difficult to name biomarkers at this point. It is important to implement quality assurance as an important component of future diagnostics at the right time. This also includes the comparability and harmonization of results from different providers of these tests. For a significant amount of tests that are routinely used and have considerable diagnostics and therefore clinical relevance, these improvements are still pending. Quality characteristics of the tests are also important for the clinical implementation of new procedures.
What requirements do you place on the manufacturers of diagnostic tests and quality controls?
Prof. Uhr:
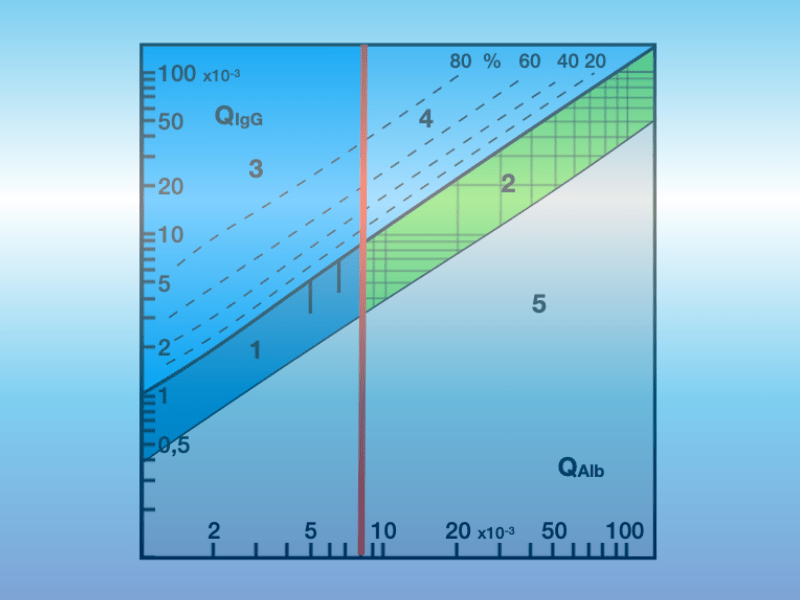
Tests and quality controls must meet the characteristics of CSF analysis. This means that individual measurements must be placed in the context of the report. An example for this are the immunoglobulin quotients relevant for the diagnostic conclusion. Individual values in CSF are irrelevant, reference ranges for individual CSF values are incorrect. The measurements should also be set up in such a way that this is taken into account in order to determine the quotient as accurately as possible. Also, the respective analyte in CSF and serum with the identical test from one manufacturer should be measured in the same concentration range on the same standard curve. This balances technical and biological factors in the quotient.
The same applies to quality controls. Here too, what is clinically relevant should be reviewed. Meaning, that quality controls should ideally be designed in a way that they correspond to clinical reality and represent a significant finding for CSF and serum. This should be assessed as relevant for internal and external quality control. Unfortunately, for whatever reason, there is a considerable need to catch up.
The use of point of care devices is becoming increasingly important. How can the best possible quality of laboratory results be ensured?
Prof. Uhr:
Independent of the use of point of care devices, the most important factor for good quality findings, at least in CSF analysis, is the specialist knowledge of the staff performing the analysis. Understanding the complexity and interrelationships of the individual CSF parameters is what makes a technical and medical plausibility check possible. Of course, this can only be achieved on the basis of very reliable measurements that are sufficiently accurate for the respective investigation.
What challenges do you see in the implementation of the new European in-vitro diagnostic medical devices Regulation (IVDR, Regulation (EU) 2017/746) in everyday laboratory work?
Prof. Uhr:
The new IVDR does not necessarily contribute directly to an improvement in quality. It is a challenge particularly for CSF analysis, since it is not uncommon to use tests developed in the laboratory or at least are used in the laboratory without sufficient validation in accordance with the IVDR. In addition, considerable organisational effort is also required for manufacturers to bring new tests or control material into the market. This has at least led to the fact that currently no CSF control material with CSF matrix has yet been approved, because the associated effort for sales was too high for the companies.
How will digital diagnostics and the possible use of AI change everyday laboratory work and what opportunities do you see here specifically for CSF diagnostics?
Prof. Uhr:
The typical CSF evaluation conditions, which are determined not least by anatomy and physiology and only lead to meaningful CSF findings in their complexity, will initially not be able to be replaced by AI alone in the everyday laboratory work. It remains to be seen, to what extent complementary AI will bring additional benefits in the case of supplementary clinical information. At present, it would be pleasing if at least an integrated CSF data report were to emerge.
Prof. Uhr, thank you very much for the interesting conversation on the subject of “Quality control in CSF diagnostics”.
We look forward to seeing you at the 10th CSF Symposium on May 4th, 2024 in Dresden. The sharing of experiences between physicians, laboratory technicians and IVD manufacturers offers the opportunity to constantly improve the quality of laboratory medical examinations.
The interview was led by Dr. Dagmar Michel

Biologist Dr. Dagmar Michel
The interview “Quality control in CSF diagnostics – Standard or economy-based?” was performed by Dr. Dagmar Michel with Prof. Dr. Dr. Uhr, Head of the Clinical Laboratory of the Max Planck Institute of Psychiatry in Munich. Dr. Dagmar Michel holds a degree in biology and has been working as a Medical Device Consultant at BIOMED Labordiagnostik GmbH for over 13 years. She is mainly responsible for products in the field of clinical chemistry and in particular for quality controls in the CSF diagnostics. Previously, Dr. Michel worked in tumor research at the LMU Klinikum on the Großhadern campus.